44 cautionary and advisory labels for medicines
Hydrocortisone with benzalkonium chloride, dimeticone and nystatin ... There can be variation in the licensing of different medicines containing the same drug. ... Cautionary and advisory labels. Label 28 . Spread thinly on the affected skin only. Taenwch yn denau ar y croen sydd wedi'i effeithio yn unig. Excipients. May contain butylated hydroxyanisole, cetostearyl alcohol (including cetyl and stearyl alcohol ... Medicinal forms | Bilastine | Drugs | BNFC | NICE Cautionary and advisory labels. Label 23 . Take this medicine when your stomach is empty. This means an hour before food or 2 hours after food. Cymerwch y feddyginiaeth hon ar stumog wag. Mae hyn yn golygu awr cyn, neu 2 awr ar ôl bwyd. Show Ilaxten 20mg tablets A. Menarini Farmaceutica Internazionale SRL Show Cautionary and advisory labels ...
Bagot Press - Pharmacy Consumables & Print Specialists PHARMACEUTICAL >, Cautionary and Advisory Labels, The Bagot Press Cautionary and Advisory label system is one of the few approved licences used for printing labels for prescribed medicines and the bright colour labels draw attention to important specific information to patients. Ordering Guide,

Cautionary and advisory labels for medicines
Cautionary Advisory Labels (CAL) - Medi Print Do Not Take Alcohol - CAL, Take on an empty stomach - Half hour before meals and at bedtime - CAL, Take on an empty stomach - Half hour before or two hours after food - CAL, Ask your doctor or pharmacist before taking medicines for heartburn - CAL, Do not take dairy products - CAL, Cautionary and advisory labels | About | BNF | NICE Pharmacists will be aware (from a knowledge of physiology) that the usual time during which indigestion remedies should be avoided is at least 2 hours before and after the majority of medicines have been taken; when a manufacturer advises a different time period, this can be followed, and should be explained to the patient. Label 6, Label Statements Database - Medsafe Unless specifically indicated, the statements do not apply to prescription medicines. Unless indicated otherwise, the label statements are required for all products containing any proportion of the named ingredient or any form of that ingredient. In this database "For oral use" means the product is intended to be swallowed.
Cautionary and advisory labels for medicines. PDF Revisions to APF24 Cautionary advisory labels Label 12 is recommended for medicines with the potential to cause CNS disturbances (e.g. dizziness, light-headedness, fatigue) and impair psychomotor performance. These symptoms frequently disappear with continued therapy. However, certain individuals (e.g. the elderly or patients taking multiple medicines) may be more susceptible to these effects. Warning statements for labels and leaflets of certain medicines Warning statements need to be added to the labels and leaflets of certain medicines. The words in this guidance do not need to be used verbatim but that have already been user tested and are in ... Outcome of the consultation on the proposed warning and advisory ... The Label Statements Database will be updated on 1 September 2022. Medicines released for supply in New Zealand after 1 March 2024 must have updated package labels that include the new warning and advisory statements. However, we encourage sponsors to update their labels before this date, if feasible. Medicinal forms | Oxycodone hydrochloride | Drugs | BNF | NICE There can be variation in the licensing of different medicines containing the same drug. Forms available from special-order manufacturers include: oral solution, solution for infusion. Tablet. All products. Show Cautionary and advisory labels. Label 2 . Warning: This medicine may make you sleepy. If this happens, do not drive or use tools or machines. Do not drink alcohol . Rhybudd: …
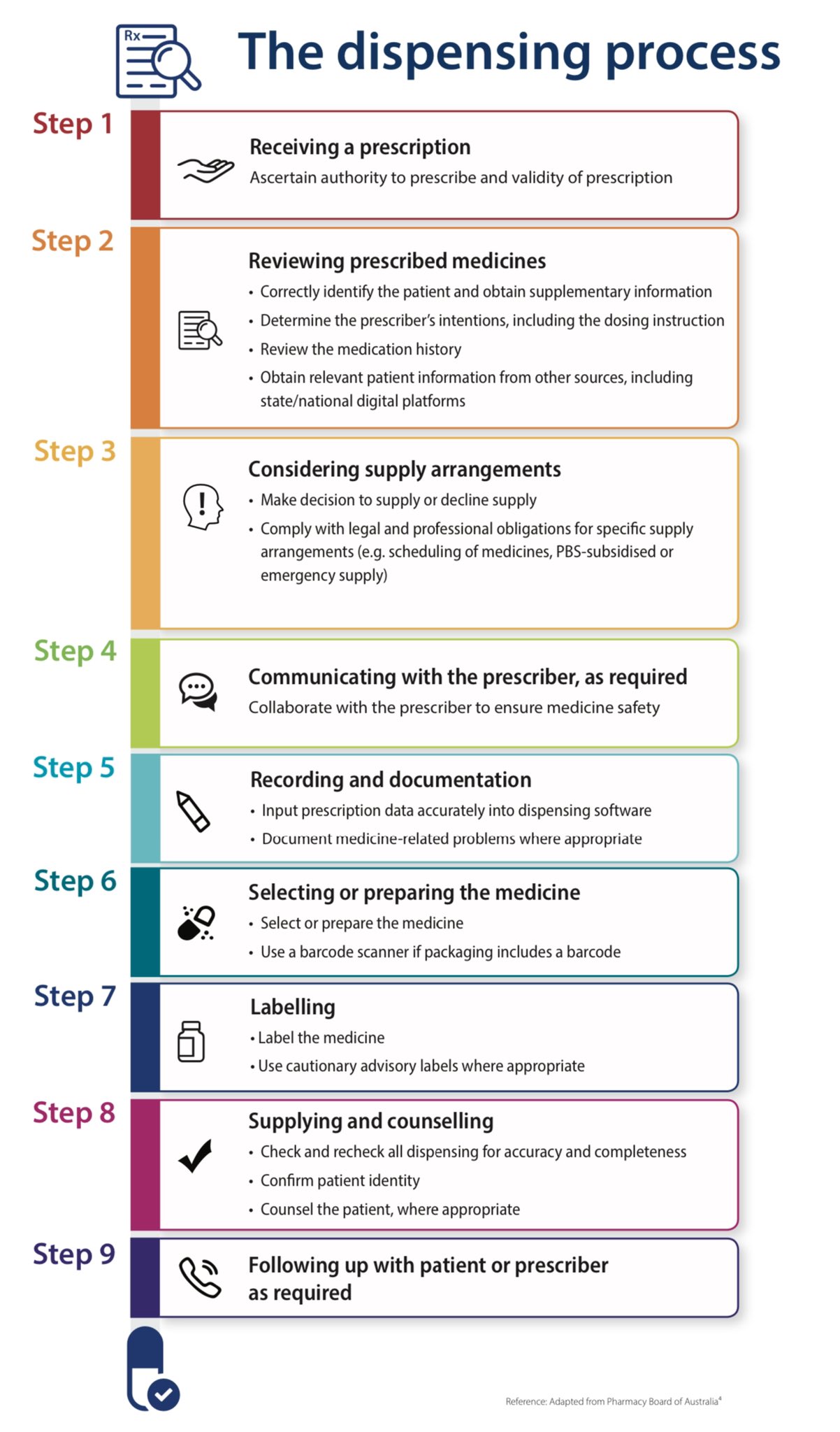
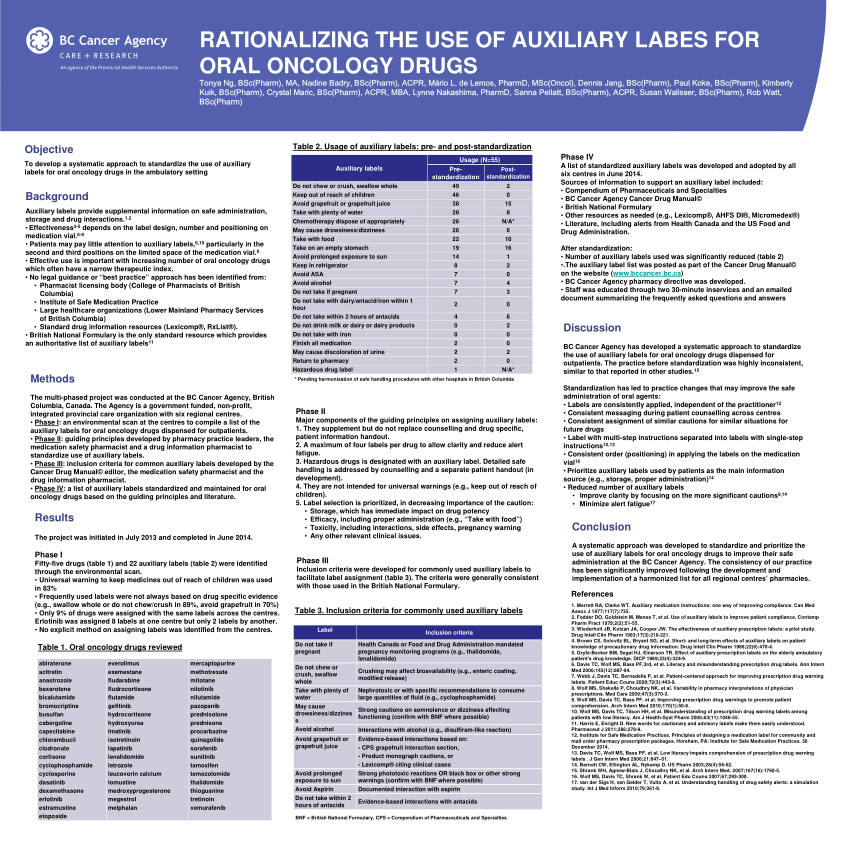
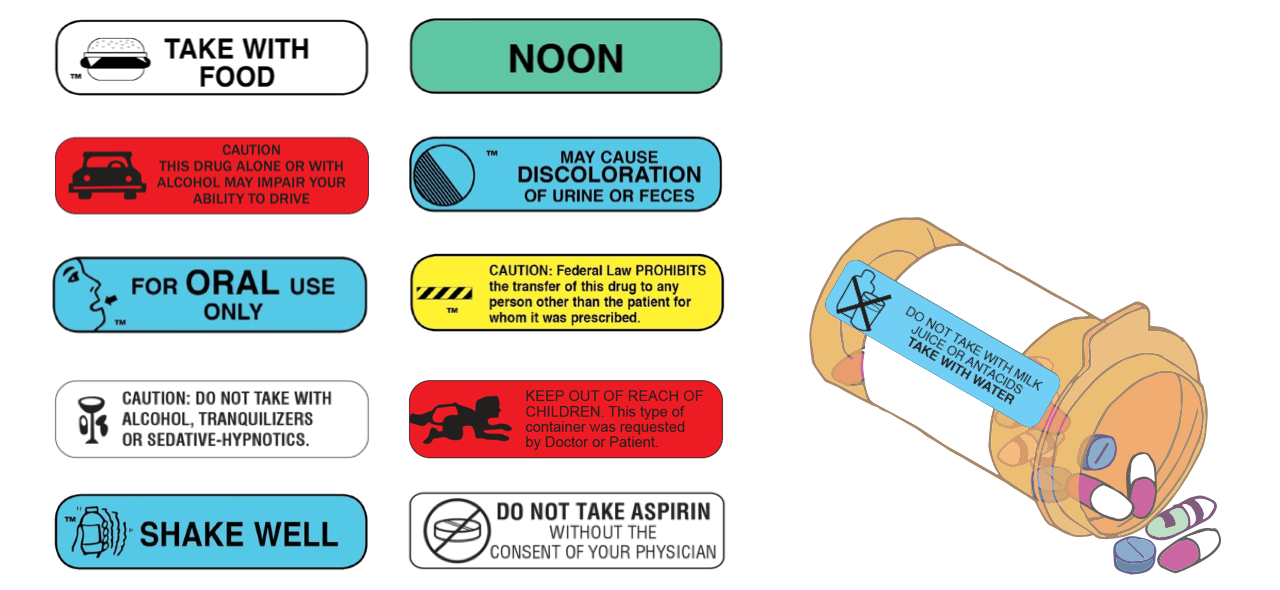
Cautionary And Advisory Labels - Medico Pak Cautionary And Advisory Labels, These labels are available in eye-catching fluro orange with different statements or instructions. We recommend using these labels to assist care facilities to maximise the safety by affixing appropriate labels to the pack where necessary. Labels measure 40mm x 20mm. Auxiliary label - Wikipedia An auxiliary label (also called cautionary and advisory label or prescription drug warning label) is a label added on to a dispensed medication package by a pharmacist in addition to the usual prescription label. These labels are intended to provide supplementary information regarding safe administration, use, and storage of the medication. [1] , Medicinal forms | Tapentadol | Drugs | BNFC | NICE Oral solution, There can be variation in the licensing of different medicines containing the same drug. Oral solution, All products, Show, Cautionary and advisory labels, Excipients, May contain propylene glycol. Show, CD2 Sugar free Palexia 20mg/ml oral solution Grunenthal Ltd, Back to top, BNFC (British National Formulary for Children) | NICE 31.08.2022 · Medicines approved by the NHS for Nurse Practitioner prescribing. Dental practitioners formulary . Medicines approved by the NHS for dental prescribing. Approximate conversions and units. Conversions and units tables. Includes growth chart with average weight and height, by gender and age (neonate, child and adult). Cautionary and advisory labels. …
Medicinal forms | Peanut protein | Drugs | BNF | NICE There can be variation in the licensing of different medicines containing the same drug. Powder. All products. Show Cautionary and advisory labels ... Show Cautionary and advisory labels. Label 21 . Take with or just after food, or a meal. Cymerwch gyda neu ar ôl bwyd. Active ingredients. Peanut protein (as defatted powder of Arachis hypogaea ... Cautionary and advisory labels for dispensed medicines - Blogger Cautionary and advisory labels for dispensed medicines, Numbers following the preparation entries in the BNF correspond to the code numbers of the cautionary labels that pharmacist are recommended to add when dispensing. It is also expected that pharmacists will counsel patients when necessary. Medicine labels - what do the warning statements mean? | Health ... All tablets and capsules should be taken with water. Some medicines can cause stomach problems, indigestion and problems with your throat and oesophagus (the pipe between your throat and stomach) if they are not swallowed properly. Taking them with a large glass of water helps to wash them down. Safer dispensing labels for prescription medicines Dispensing labels on prescribed medicines provide administration instructions and important warnings. These remain with the consumer after the initial consultation when some of the confusion and worry frequently associated with illness has started to recede. ... Australia has a national 'standard icon system' for cautionary advisory labels ...
Cautionary advisory labels - Australian Pharmacist The dispensing pharmacist has dispensed the patient's discharge medications and has labelled simvastatin with cautionary advisory label 21 (Special handling and disposal required - ask your pharmacist) and label A (Swallow whole do not crush or chew). You are concerned that these labels may alarm and/or confuse the patient. What should you do?
Medicinal forms | Macrogol 3350 with potassium chloride, sodium ... There can be variation in the licensing of different medicines containing the same drug. Oral solution . All products. Show Cautionary and advisory labels. Label 13 . Dissolve or mix with water before taking. Gadewch i doddi mewn dŵr cyn ei gymryd. Electrolytes. May contain bicarbonate, chloride, potassium, sodium. Show Sugar free Movicol Liquid Forum Health Products Ltd Show …
Labels on medicines and poisons - Department of Health Some medicines also require additional label warnings. For example, oral retinoids must have warnings about becoming pregnant. Sedation warnings. Medicines listed in Appendix K of the SUSMP (exernal site) must be labelled with a sedation warning when supplied to patients. Pharmacy cautionary advisory label 1 or label 1A should be used.
Medicinal forms | Betamethasone with clotrimazole | Drugs | BNFC ... Cautionary and advisory labels. Label 28 . Spread thinly on the affected skin only. Taenwch yn denau ar y croen sydd wedi'i effeithio yn unig. Excipients. May contain benzyl alcohol, cetostearyl alcohol (including cetyl and stearyl alcohol), propylene glycol. Show Lotriderm cream Organon Pharma (UK) Ltd Show Cautionary and advisory labels ...
PDF cautionary advisory labels - Openbook Howden cautionary advisory labels, OBH 18642, CAL's are a valuable tool for helping pharmacists to fulfil their legal and professional obligations, promote quality use of medicines, and provide optimal outcomes for consumers. Reproduced with the permission of the Pharmaceutical Society of Australia. Sold in dispenser boxes of 1000.
Boxed warning - Wikipedia In the United States, a boxed warning (sometimes "black box warning", colloquially) is a type of warning that appears on the package insert for certain prescription drugs, so called because the U.S. Food and Drug Administration specifies that it is formatted with a 'box' or border around the text. The FDA can require a pharmaceutical company to place a boxed warning on the labeling …
StirlingFildes | Printing, Packaging and Consumables Cautionary & Advisory Labels. Buy a mixture of 12 or more labels and save. The savings will be calculated on your invoice. Product Image Product Description & Code Product Details; Warning Label No. 1 Code: WARN 1: 1,000 per box 46 x 16mm: Warning Label No. 1A Code: WARN 1A:
New Label 24 to help pharmacists reduce opioid risks June 24, 2020. In line with Therapeutic Goods Administration (TGA) regulatory changes to enhance medicine safety, PSA has developed a cautionary advisory label (CAL) that warns consumers about the risk of opioid overdose and dependence. The CAL (Label 24, at right) can be applied to opioid medicines at the time of dispensing as an aid to ...
Colecalciferol with calcium carbonate Medicinal forms There can be variation in the licensing of different medicines containing the same drug. Tablet. All products. Excipients. May contain propylene glycol. Show Accrete D3 tablets Thornton & Ross Ltd. Active ingredients. Calcium carbonate 1.5 gram, Colecalciferol 400 unit . Size 60. Unit tablet. NHS indicative price £2.95. Drug tariff Part VIIIA Category C. Drug tariff price £2.95. Legal ...
Required Advisory Statements for Medicine Labels (RASML) Australian labelling requirements for non-prescription medicines ( Therapeutic Goods Order No. 92) require some over-the-counter and complementary medicine labels to contain particular warning statements ('advisory statements') about specific risks related to use of the medicines.
Medicinal forms | Quetiapine | Drugs | BNF | NICE Cautionary and advisory labels. Label 2 . Warning: This medicine may make you sleepy. If this happens, do not drive or use tools or machines. Do not drink alcohol. Rhybudd: Gall y feddyginiaeth hon eich gwneud yn gysglyd. Peidiwch â gyrru, defnyddio offer llaw neu beiriannau os yw hyn yn digwydd. Peidiwch ag yfed alcohol. Label 23
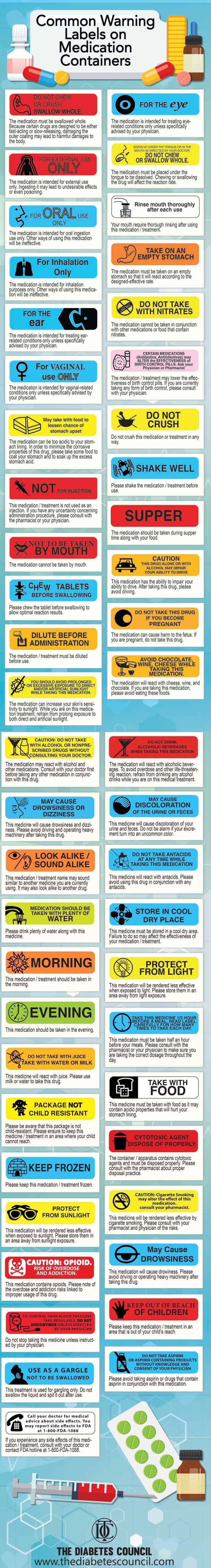
50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings. The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even ...
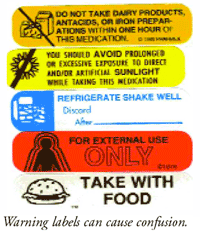
Cautionary and advisory labels for medicines - SlideShare Standard cautionary and advisory labels offer advice but are not exhaustive. The labels are not a substitute for adequate counseling by prescribers and dispensers (most medicines are dispensed by pharmacists) but are intended to reinforce essential information the patient needs to know. Kiran Sharma, KIET School of Pharmacy 11/12/2013 2,
Cautionary_and_advisory_label - chemeurope.com Cautionary and advisory label, Cautionary and advisory labels (Cals) are sometimes added (with the dispensing label) to a medicine dispensed by the pharmacist to the patient. Daily Visual Balance Check, Recognize and detect the effects of electrostatic charges on your balance, Better weighing performance in 6 easy steps,
Cautionary and advisory labels for medicines - Everything2.com This medicine may colour the urine, To be used on preparations that may cause the patients urine to turn an unusual colour, Caution flammable: keep away from fire or flames, Allow to dissolve under the tongue. Do not transfer from this container. Keep tightly close. Discard eight weeks after opening, To be used on glyceryl trinitrate tablets,
Formulary A3 - Cautionary and advisory labels: A4 - Intravenous additives: A5 - Wound management: B2 - Borderline substances: P - Prescribing information: Show/Hide Keys To Symbols Latest News Latest News Welcome to the Inform Formulary. Swansea Bay UHB Formulary This formulary lists all medicines approved for use in Swansea Bay UHB (for both Primary and Secondary care). …
Medicinal forms | Selumetinib | Drugs | BNFC | NICE Cautionary and advisory labels. Label 25 . Swallow this medicine whole. Do not chew or crush. Llyncwch yn gyfan. Peidiwch â chnoi neu falu'n fân. Excipients. May contain vitamin e. Show (black triangle) Koselugo 10mg capsules AstraZeneca UK Ltd Show Cautionary and advisory labels. Label 25 . Swallow this medicine whole. Do not chew or crush ...
Consumer Updates | FDA - U.S. Food and Drug Administration 28.07.2022 · The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
StirlingFildes | HealthCare Cautionary Advisory Labels, The StirlingFildes Cautionary Advisory Label (CALs) system produced by the PSA is a valuable, labelling system designed for helping pharmacists to fulfil their legal and professional, obligations, promote quality use of medicines, and provide optimal outcomes for consumers.
British National Formulary - Wikipedia The British National Formulary (BNF) is a United Kingdom (UK) pharmaceutical reference book that contains a wide spectrum of information and advice on prescribing and pharmacology, along with specific facts and details about many medicines available on the UK National Health Service (NHS). Information within the BNF includes indication(s), contraindications, side effects, doses, …
Outcome of the consultation on the proposed warning and advisory ... The Label Statements Database will be updated on 1 September 2022. Medicines released for supply in New Zealand after 1 March 2024 must have updated package labels that include the new warning and advisory statement. However, we encourage sponsors to update their labels before this date, if feasible.
Label Statements Database - Medsafe Unless specifically indicated, the statements do not apply to prescription medicines. Unless indicated otherwise, the label statements are required for all products containing any proportion of the named ingredient or any form of that ingredient. In this database "For oral use" means the product is intended to be swallowed.
Cautionary and advisory labels | About | BNF | NICE Pharmacists will be aware (from a knowledge of physiology) that the usual time during which indigestion remedies should be avoided is at least 2 hours before and after the majority of medicines have been taken; when a manufacturer advises a different time period, this can be followed, and should be explained to the patient. Label 6,
Cautionary Advisory Labels (CAL) - Medi Print Do Not Take Alcohol - CAL, Take on an empty stomach - Half hour before meals and at bedtime - CAL, Take on an empty stomach - Half hour before or two hours after food - CAL, Ask your doctor or pharmacist before taking medicines for heartburn - CAL, Do not take dairy products - CAL,



























Post a Comment for "44 cautionary and advisory labels for medicines"